views
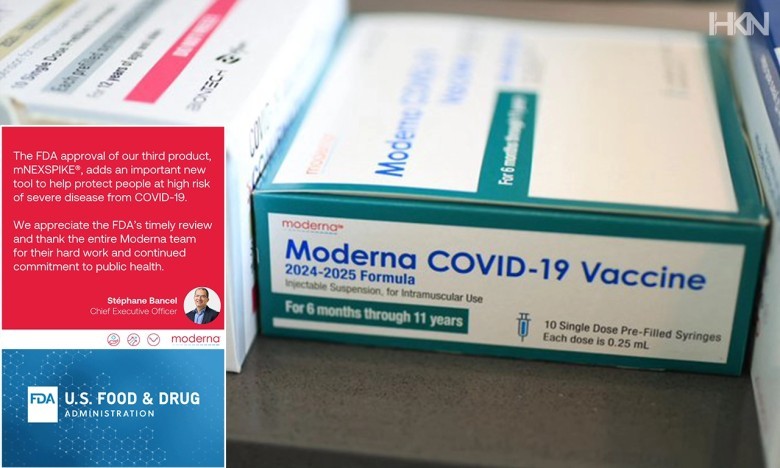
The U.S. Food And Drug Administration has officially approved Moderna’s next-generation COVID-19 vaccine for people aged 65 or older. The approval was granted following a thorough review under the agency’s updated requirements for vaccines targeting high-risk populations.
This marks a major step in preparing for upcoming seasonal outbreaks. Moderna developed the vaccine using mRNA technology to better target the latest coronavirus variants and to provide longer immunity for older adults. The vaccine showed strong results in clinical trials among older adults who typically have a weaker immune response.
A Targeted Move To Protect America’s Most Vulnerable Population
The company said the updated shot demonstrated improved protection against the latest strains circulating in the U.S. and showed fewer side effects compared to previous versions. Federal health officials are encouraging eligible individuals to receive the new shot before the end of the year.
The FDA’s decision is seen as part of a wider public health push to shield seniors who are at higher risk of severe illness and hospitalization. This approval follows recent concerns that immunity among older adults is declining faster than expected. Moderna said the shot will be available in pharmacies nationwide starting next month. Health systems and senior care centers are also preparing mass vaccination plans for the coming months.
"The approval of this updated vaccine helps ensure that the most vulnerable population receives the best possible protection against COVID-19," a senior health official said.
Experts expect similar approvals for updated shots from other vaccine makers soon. The Centers for Disease Control and Prevention is expected to issue formal guidance for distribution and eligibility next week.
















![UC Davis Showdown: Student Shuts Down Hater with Powerful Defense of Trans Community [WATCH]](/upload/media/posts/2025-04/11/uc-davis-showdown-student-shuts-down-hater-with-powerful-defense-of-trans-community-watch_1744363407-s.jpg)


